Abstract
Invasive fungal disease (IFD) is a major cause of morbidity and mortality in patients treated for haematological malignancies. The major determinants of outcome are the degree of the patient's immune incompetence, the fungal species and the presence of drug resistance in the infecting organism. As uncommon and drug resistant strains increase in frequency, effective alternative treatment strategies are needed to improve the dismal outcomes for IFD occurring after hematopoietic stem cell transplant (HSCT). Recent data provide strong evidence that a therapeutic benefit is seen using adoptive transfer of cryopreserved, partially human leukocyte antigen (HLA) matched 3rd party T-cells for post-transplant viral infections in HSCT recipients. We propose that a similar approach may be effective in IFDs.
In the present study, we developed a method for rapid manufacture of large numbers of broadly reactive and highly specific antifungal T-cells starting with a stem cell product obtained from healthy donors as part of normal allogeneic transplant procedure. Water-soluble lysates of germinated conidia of Aspergillus and Candida species were used to stimulate 60.5 ± 21.7 x 106 mononuclear cells from the hematopoietic precursor cell (HPC) product. Reactive T-cells were selected using activation marker and magnetic bead isolation and expanded in culture with cytokines. After elution from the magnet, the enriched population measured 82.5±13.3 x103 cells of which 82.3±6.9% were CD4+ (n=6). All cultures were maintained for a total of 11 to 12 days, leading to 126.8±11.9x106 fungus-specific T cells, corresponding to approximately 1000 fold expansion within 12 days of culture initiation. At the end of culture greater than 97.3±0.9% of cells were CD3+, of which 91.7±1.2% were CD4+ cells and 3.6±0.7% were CD8+ cells.
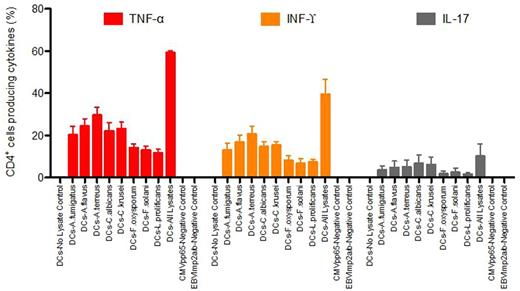
Responses against fungus were measured using cytokine flow cytometry after restimulation by specific fungal lysates. 59.4±0.9% of CD4+ cells were reactive against fungus-primed monocyte-derived dendritic cells (MoDCs). Production of the pro-inflammatory cytokines tumour necrosis factor (TNF)-α, interleukin (IL)-17 and interferon (IFN)-γ following challenge showed that the expanded T-cell populations had broad reactivity against Aspergillus fumigatus / flavus / terreus, Candida albicans / krusei, Fusarium oxysporum / solani and Leptosporum (Scedosporium) prolificans (Figure 1).
We previously demonstrated that antifungal reactivity is mediated through HLA-DR and can occur through a single matching HLA-DR molecule. We calculated that in our HSCT population, donors with the seven highest frequency HLA-DR molecules (DRB1 1:01, 3:01, 4:01, 7:01, 11:01, 13:01 or 15:01) could be used to generate a bank of cryopreserved fungus-specific T lymphocytes to treat IFDs in HSCT recipients. Using a bank of this size, approximately 85% of HSCT recipients would have a fungus-specific T cell product in the bank matching at least 1 HLA-DR molecule. Increasing the bank to add donors with four additional HLA-DR types (DRB1 4:04, 11:04, 13:02 and 15:02) would increase the percentage of recipients with a banked product matching at least 1 of HLA-DR molecules to almost 95%.
In conclusion, we have identified a GMP compliant method to rapidly manufacture fungal T-cell products to high number with specificity against a range of clinically relevant fungal pathogens including yeasts and moulds. Using these cells, we intend to construct a bank of fungus-specific T cells from donors with common HLA-DRB1 types and to test their efficacy in HSCT recipients with IFD. If effective in restoring immunity, adoptive cell therapy using 3rd party broadly reactive antifungal T-cells could be useful in improving outcomes for HSCT patients with IFDs.
Figure 1: Cytokine expression profile of reactive antifungal T cells expanded with Aspergillus and Candida lysates following rechallenge with lysates of specific fungal pathogens or a mix of all lysates. Results are means of 6 experiments (±s.e.m) and shown as percentage of CD4+ T cells.
Gottlieb: Indee: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal